lncRNA: Biomarker for diagnosing and prognosing cardiovascular diseases
Cardiovascular diseases (CVDs) are the leading cause of death globally. According to the World Health Organisation, an estimated 17.9 million people die each year from CVDs. This represents 32% of all global deaths. CVDs are responsible for twice as many death as cancer in both men and women.
Among CVDs, heart failure is the most prevalent. Heart failure is a chronic, progressive condition in which the heart muscle does not pump blood as well as it should, unable to keep with its workload. An estimated 26 million people suffer from heart failure each year, and there are 600,000 new cases of heart failure each year in Europe. Experts predict that the prevalence of heart failure and the associated healthcare costs will steadily increase over the next 15 years.


While the management of CVDs, heart failure, in particular, has improved in the past decade, there is still a need for novel and highly efficient strategies to treat CVDs. The high prevalence of CVDs can be addressed through the benefits personalised medicine offers. Personalised medicine, also known as precision medicine, is a medical model whereby treatment and intervention are tailored to the individual patient based on their predicted response or risk of disease. For example, patients at high risk of developing CVDs can be identified using biomarkers.
What are biomarkers?
A biomarker is a substance that is measured in a biological system as an indicator of an underlying condition or disease. Biomarkers are easily detectable, measurable and they provide robust information on the state of disease. There are different types of biomarkers ranging from proteins, lipids, small molecules, and nucleic acids (DNA and RNA). In the context of the COVIRNA project, the biomarker used is called IncRNA.
What is lncRNA?
All life is fundamentally made up of the same building blocks – DNA, deoxyribonucleic acid, and RNA, ribonucleic acid. While DNA replicates and stores genetic information, RNA converts the genetic information contained within DNA to a format used to build proteins. RNA molecules are classified into two groups, coding RNAs and non-coding RNAs. Coding RNAs are the messenger RNAs that are able to induce the production of proteins. Non-coding RNAs on the other hand are unable to encode proteins.
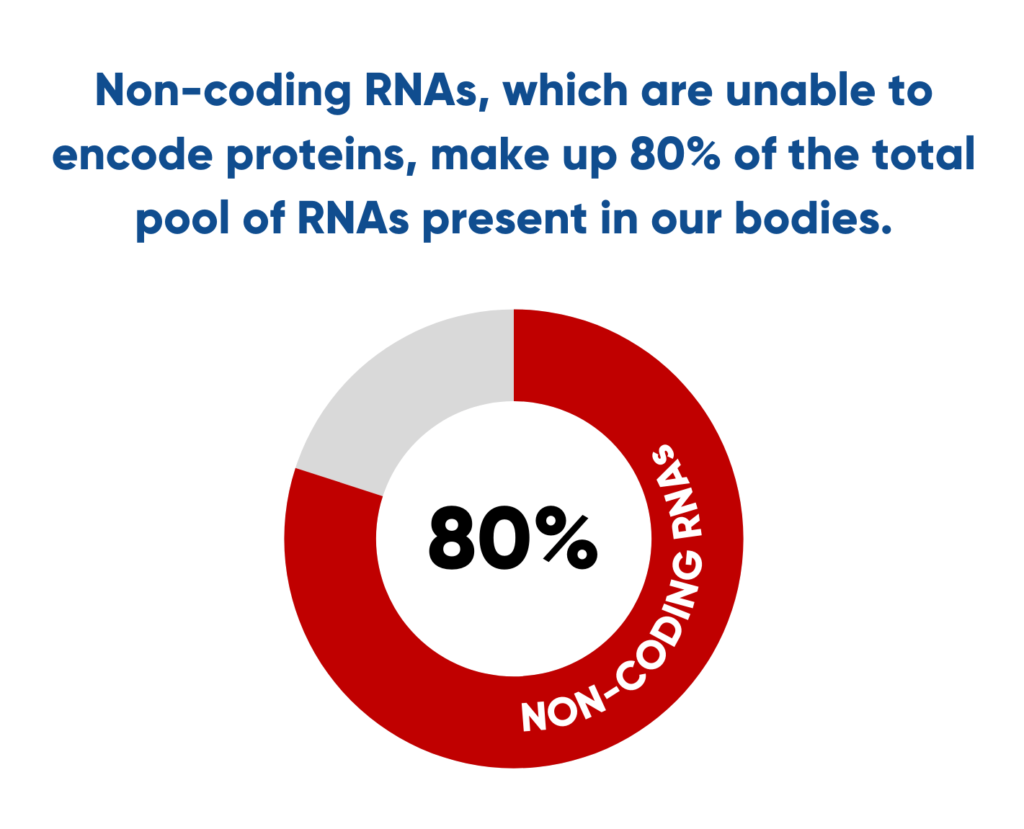
Not too long ago, scientists thought of RNAs as only a simple short-lived copy of our DNA, serving as a template for the synthesis of proteins. However, recent research suggests that the vast majority of RNAs are not able to encode proteins. More precisely, non-coding RNAs make up 80% of the total pool of RNAs present in our bodies.
Non-coding RNAs are divided into different sub-groups one of which is lncRNA, long non-coding RNA. lncRNAs are RNA molecules with more than 200 nucleotides, unable to encode proteins. lncRNAs are generating a lot of interest from scientists because they can play a significant role in our biology:
• cell development and differentiation;
• epigenetic regulation, i.e. altering the physical structure of DNA;
• pathogenic disease processes where they are identified as new biomarkers or druggable targets for cancer, diabetes, heart disease, and inflammation.
lncRNAs play a key role in the occurrence and development of myocardial infarction, heart failure, myocardial hypertrophy, arrhythmias and other pathological processes that significantly affect the prognosis and survival of patients with cardiovascular diseases.
How will lncRNA be used in the COVIRNA project?
The COVIRNA project is a patient-centred Innovation Action bringing together 15 European partners to develop a tool to identify COVID-19 patients at risk of developing fatal cardiovascular complications in the context of the current pandemic, with the goal of improving individualised surveillance, care, and follow-up of patients.
To achieve this goal, COVIRNA will build a biobank of 2,000 blood samples from existing cohorts of COVID-19 patients throughout Europe to perform a multi-centre international study. COVIRNA will then identify a subset of highly specific lncRNAs that are predictive of COVID-19 clinical outcomes using bioinformatics, Artificial Intelligence and biostatistics. COVIRNA will specifically look at the following disease outcomes: survival, as a primary end-point, and myocardial infarction, coronary revascularisation, stroke, and hospitalisation for heart failure, as a secondary end-point. lncRNAs identified as best predictors of outcome will be investigated for their functional association with the disease and its progression. The COVIRNA Consortium will provide a unified and coordinated effort to discover novel functions of lncRNAs and potentially identify novel therapeutic targets.
Using lncRNAs and clinical data collected, COVIRNA will build a disease evolution prediction model. This will set the stage for the development of a reliable, cost-efficient and easy-to-use in vitro diagnostic test to predict COVID-19 clinical outcomes.
To learn more about the COVIRNA project:
» Watch the official video of the COVIRNA project
» Download the COVIRNA project flyer
» Subscribe to the COVIRNA project newsletter
