About the Project
The Goal
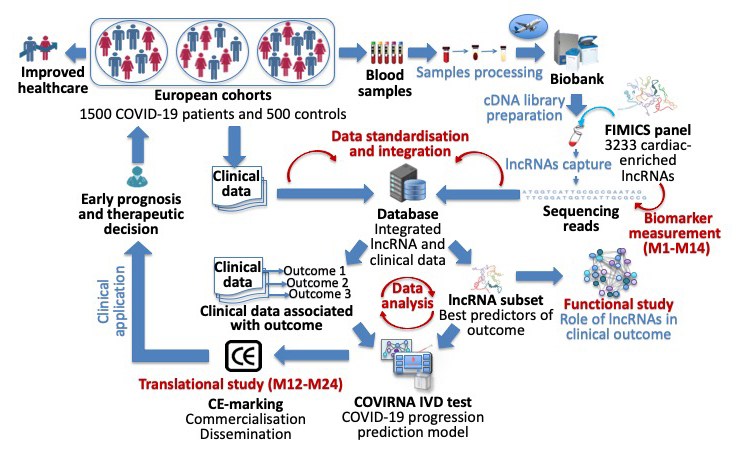
The overall goal of the COVIRNA project is to generate a diagnostic test based on cardiovascular RNA biomarkers highly predictive of the clinical outcomes of COVID-19 patients and to enable its rapid market uptake with the aim to improve individualised surveillance, care and follow-up of these patients in the context of the current pandemic.
Objective: At the technological and research levels
- To build a biobank of 2,000 blood samples from existing cohorts of COVID-19 patients throughout Europe to perform a retrospective multicentre international study;
- To achieve biomarker qualification and select a subset of highly specific lncRNAs predictive of COVID-19 clinical outcome using bioinformatics, artificial intelligence (AI) and biostatistics;
- To build a disease evolution prediction model based on selected lncRNAs and clinical data;
- To design a reliable, cost-efficient and easy to use in vitro diagnostic (IVD) test to predict COVID19 clinical outcomes.

Objective: At the socio-economic and regulatory levels
- To achieve CE marking of the innovative COVIRNA prognostic solution;
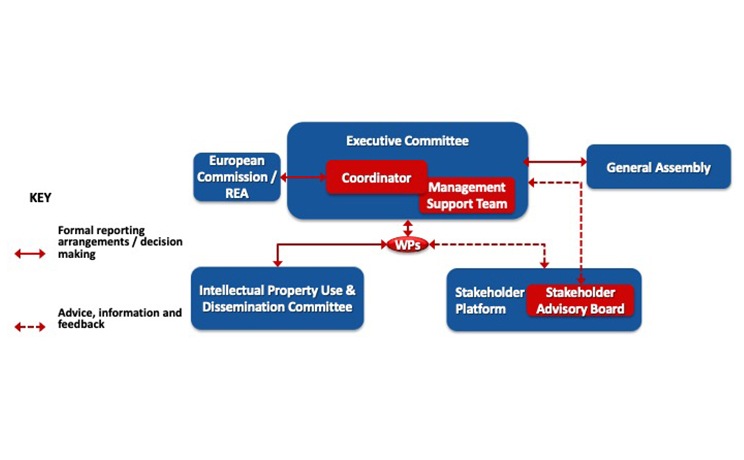
- To establish a strategic science-policy-business-society consultation to optimize the design of the diagnosis solution complying with end-users’ needs, cost-efficiency analysis requirements, current EU regulation and highest quality standards to enable and accelerate their uptake into clinical practice;
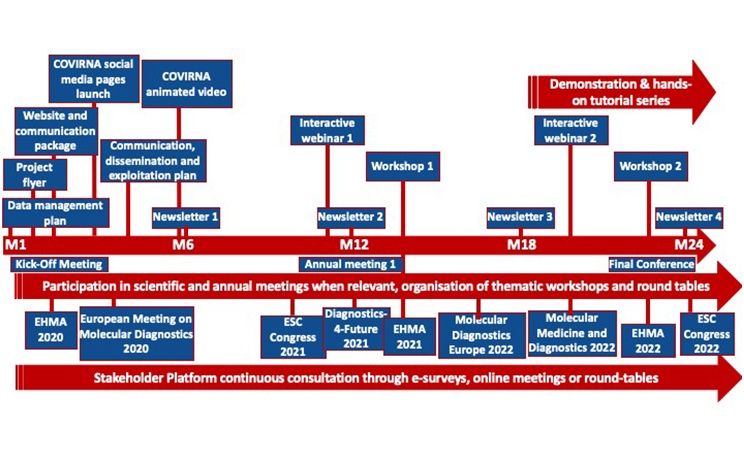
- To raise stakeholders’ awareness of advantages brought by the newly designed diagnostic solution as a valuable decision-support tool for healthcare professionals to deliver the best health outcome for the most vulnerable COVID-19 patients, through a tailored dissemination programme;
- Engage communities of stakeholders in sharing practical knowledge on the use of the novel medical technology.